- HOME
- Members
- Principal Investigators
- Principal Investigators
- Goro Sashida
RESEARCH
Laboratory of Transcriptional Regulation in Leukemogenesis
Research Field:
Hematopoietic stem cell, Transformation, Transcription factor, Chromatin and Epigenome.
Research Interests:
To understand how hematopoietic stem cells develop myeloid malignancies: Myelodysplastic syndrome (MDS) is a group of clonal disorders of hematopoietic stem cells characterized by ineffective hematopoiesis, peripheral blood cytopenias, dysplastic blood cells and a predisposition to acute myeloid leukemia (AML). Given that MDS cells are phenotypically and genetically heterogeneous, the molecular mechanism of the development of MDS is incompletely understood. Recent genome sequencing studies have identified various somatic mutations of epigenetic modifiers in patients with myeloid malignancies. Interestingly, the mutations in epigenetic modifiers, which were often seen in MDS cells, were also identified in blood cells of healthy aged adults. Indeed, aging is known to increase the risk of myeloid malignancies including MDS, conceivably due to environmental stress-induced genetic and/or epigenetic alterations. Therefore, the accumulation of epigenetic alterations appears to provide pre-MDS stem cells and facilitate the initiation and propagation of MDS. Our laboratory is working to determine how genetic and epigenetic alterations collaborate to promote the development of myeloid malignancies by utilizing comprehensive approaches (e.g. genetically modified mice, CRISPR/Cas9 genome editing, and sequencing analysis such as CUT & Tag-seq, ATAC-seq, and Bisulfate-seq). We are also working on HMGA/HMGN chromatin modifers in fluke-induced bile duct cancer in collaboration with Dr. Sawanyawisuth in Khon Kaen Univeristy in Thailand and visiting students (please refer to Sawanyawisuth K, et al. Cancers 2021; Sorin S, et al. FASEB J 2022). Several areas of current investigation on malignant hematopoiesis in the lab include:
- Understanding how transcription factors and chromatin modifiers drive development of MDS and AML
I have elucidated the epigenomic regulatory mechanisms of RUNX1 transcription factor and MLL1, among others, in the United States and at Chiba University (Zhang Y et al. Blood 2012; Tanaka S, et al Blood 2012). We were the first to report the stem cell regulatory mechanism of SF3B1, a splicing factor that is frequently mutated in clonal hematopoiesis (Wang C, et al. Blood 2014). Furthermore, we established several myelodysplastic syndrome models using genetically engineered mice with EZH2 and TET2, which are frequently mutated in hematopoietic tumors, to elucidate the pathogenetic basis of the disease due to epigenomic dysregulation (Muto T, et al. J Exp Med 2013; Sashida G, et al. Nature Commun 2014; Mochizuki-Kashio M, et al. Blood 2015; Hasegawa N, et al. Leukemia 2017). At Kumamoto University, I am elucidating the pathogenetic basis of myeloid malignancies and refractory rare leukemia, focusing on chromatin dynamics and enhancer dysfunction (Sashida G, et al. J Exp Med 2016; Wang C, et al. J Clin Invest 2018; Kubota S, et al. Nature Commun 2019; Yokomizo-Nakano T, et al. Cancer Res 2020; Bai J, et al. Oncogene 2021).
- Understanding how aging and environmental stresses impair normal stem cell and drive development of myeloid malignacies
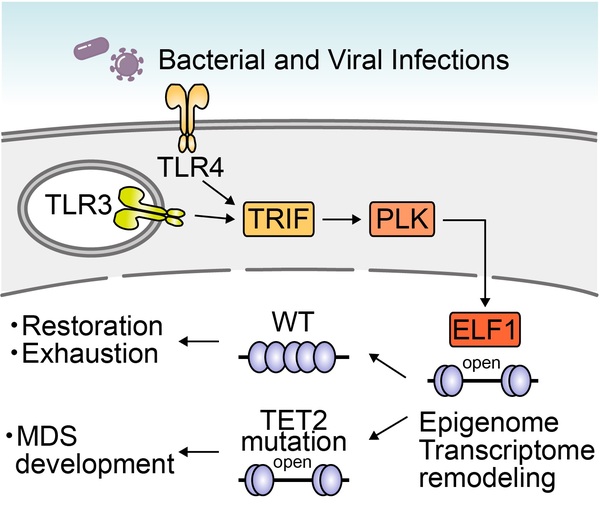
We generated several inducible leukemia models such as a clinically relevant RUNX1-ETO leukemia murine model (Abdallah MG, et al. Leukemia 2021) and study the molecular mechanisims of age-dependent development of leukemia. Recently, we demonstrated that a prior stimulation with bacterial and viral products followed by loss of the Tet2 gene facilitated the development of MDS via up-regulating the target genes of the Elf1 transcription factor and remodeling the epigenome in hematopoietic stem cells (HSCs) in a manner that was dependent on Polo-like kinases (Plk) downstream of Tlr3/4-Trif signaling, but did not increase genomic mutations. Our study also proposes the Trif-Plk-Elf1 axis as a potential target for therapeutic interventions for MDS (a model is shown below) (Yokomizo-Nakano T, et al. J Exp Med 2023).
- Understanding how Trisomy impacts on stem cell function and drives development of myeloid malignacies (e.g. Trisomy 8 and Down syndrome)
Biography:
Goro Sashida recieved his MD from Tokyo Medical University in 1996. He then undertook his PhD in medicine under the supervision of Dr. Kazuma Ohyashiki at the Department of Hematology, Tokyo Medical University in 2002. In 2005, he joined Dr. Stephan Nimer's laboratory at Memorial Sloan-Kettering Cancer Center (New York, USA; Dr. Nimer moved to Miami). In 2009, he moved to Dr. Gang Huang's laboratory at Cincinnati Children's Hospital Medical Center (Ohio, USA; Dr. Huang moved to San Antonio), where he became interested in the study of epigenetic alteration in leukemia. In 2010, he went back to Japan and joined Dr. Atsushi Iwama's laboratory at Chiba University (Chiba, Japan; Dr. Iwama moved to Tokyo). In December 2014, he established his own research group "Laboratory of Transcriptional Regulation of Leukemogenesis" at International Research Center of Medical Sciences, Kumamoto University, and in 2016 he was promoted to Professor. He lives in Kumamoto with his wife, two sons and one daughter.

