- HOME
- Members
- Principal Investigators
- Principal Investigators
- Yuta Takahashi
RESEARCH
Exploring the mechanisms underlying transgenerational inheritance of epigenetic signatures
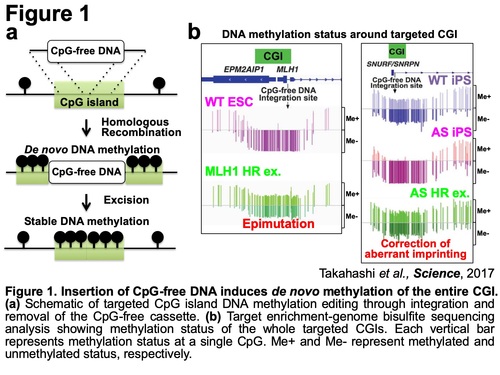
In our previous work, we have investigated the mechanism by which only CpG islands (CGIs), which are CpG-rich regions usually associated with promoter regions, remain unmethylated in the highly methylated mammalian genome. In this process, we demonstrated that insertion of CpG-free DNA, which doesn't contain any CG sequences, into targeted CGIs induces de novo methylation of the entire CGI by disrupting methylation-blocking machinery in human pluripotent stem cells (PSCs) (Figure 1) (Y. Takahashi et al., Science, 2017). The acquired methylation is stably maintained even after CpG-free DNA removal, extensive passaging, and differentiation. Thus, this approach allows for targeted CGI methylation editing. Using this approach, we have successfully generated a PSC model with cancer-related epimutation at MLH1 CGI and corrected an aberrant imprinting in Angelman Syndrome patient specific iPSCs.
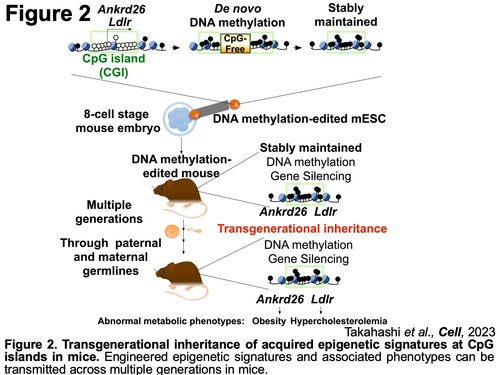
Next, we have determined an intriguing question whether transgenerational epigenetic inheritance can take place in mammals using the epigenetically modified mice generated by our unique approach. While transgenerational epigenetic inheritance is extensively documented in bacteria, protists, fungi, plants, and certain invertebrate animals, in mammals, it is generally assumed that bimodal dynamic epigenetic reprogramming in primordial germ cells (PGCs) and zygotes serves to prevent inheritance of ancestral epigenetic signatures. However, given that it has been reported that epigenome reprogramming may be incomplete and some of abnormal DNA methylation at CGIs may be transmitted from parents to their offspring in humans, it remains unclear whether epigenetic signatures can be passed to offspring in mammals. We demonstrated that DNA methylation of promoter-associated CGIs can be transmitted from parents to their offspring in mice (Figure 2) (Y. Takahashi et al., Cell, 2023). Using our unique DNA methylation editing strategies (Y. Takahashi et al., Science, 2017), we generated DNA methylation edited cells in which CGIs of two metabolism related genes, the low-density lipoprotein receptor (Ldlr) and the Ankyrin repeat domain 26 (Ankrd26), were specifically methylated, and their targeted loci were silenced. Injection of these methylated ESCs into host mouse 8-cell stage embryos enabled us to generate DNA methylation-edited mice exhibiting altered metabolic phenotypes. This approach is currently the only way to generate DNA methylation-edited mouse model, which allows for the exploration of the role of epigenetic regulation in physiological and pathological states in vivo. Moreover, the acquired methylation of the targeted CGI and the phenotypic traits were transmitted subsequent generations through both male and female germlines (Figure 2). These observations provide first direct evidence of transgenerational epigenetic inheritance in mammals, which may not only serve to increase our knowledge of mammalian embryonic development and evolution, but they may also allow for a better understanding of the etiology, diagnosis, and offspring's susceptibility of non-genetically inherited human diseases.
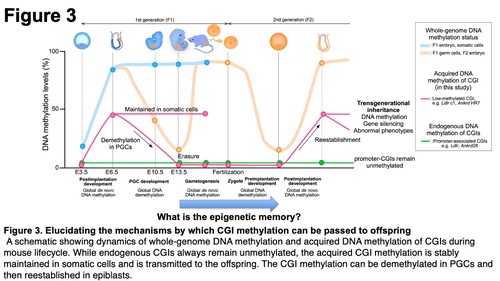
These results regarding the inheritance of methylated CGIs gave rise to a significant question about how the DNA methylation of CGIs is transmitted to offspring through parental germlines even though there is dynamic epigenetic reprogramming during development of primordial germ cells and after fertilization. We found that the heritable DNA methylation of the CGIs is erased in PGCs once and, subsequently, reestablished in next generation embryos after implantation (Figure 3), indicating that even though sperm and oocyte carry unmethylated CGI to zygote, DNA methylation can be reestablished in next generation. These results suggest that, instead of DNA methylation, sperm and oocyte carry "epigenetic memory" to zygotes, and thereby DNA methylation can be reestablished depending on the memory. Our laboratory is attempting to investigate the nature of the epigenetic memory and elucidate the mechanisms underlying transgenerational epigenetic inheritance in mammals.
Reference:
Takahashi Y, Morales Valencia M, Yu Y, Ouchi Y, Takahashi K, Shokhirev MN, Lande K, Williams AE, Fresia C, Kurita M, Hishida T, Shojima K, Hatanaka F, Nuñez-Delicado E, Rodriquez Esteban C, Izpisua Belmonte JC. Transgenerational inheritance of acquired epigenetic signatures at CpG islands in mice. Cell. 2023; 186: 4, 715-731, PMID: 36754048, DOI: 10.1016/j.cell.2022.12.047
Takahashi Y, Wu J, Suzuki K, Martinez-Redondo P, Li M, Liao HK, Wu MZ, Hernández-Benítez R, Hishida T, Shokhirev MN, Esteban CR, Sancho-Martinez I, Izpisua Belmonte JC. Integration of CpG-free DNA induces de novo methylation of CpG islands in pluripotent stem cells. Science. 2017; 356: 503-508, PMID: 28473583, DOI: 10.1126/science.aag3260