Yuta Takahashi

Tel:
096-373-6873
Fax:
E-mail:
ytakahashi[at]kumamoto-u.ac.jp
Research Field
Transgenerational epigenetic inheritance in mammals
Keywords: Transgenerational inheritance of epigenetic signatures, DNA methylation, epigenetic editing, DNA methylation editing, DNA methylation edited-mice, heritable disease, epimutation, heritable epigenetic memory
Research Interests
Epigenetic signatures refer to chemical modifications to DNA or chromatin that play a crucial role in regulating gene expression without altering the DNA sequence. While it is largely accepted that inheritance of parental traits is mainly driven by the inheritance of genomic DNA, epigenetic inheritance has been extensively documented in bacteria, protists, fungi, plants, and certain invertebrate animals, further supporting the idea that, across evolution, there is more to heredity than simply inheritance of genomic DNA. In mammals, it is generally assumed that epigenetic reprogramming during development of parental germline prevents inheritance of ancestral epigenetic signatures. However, because, for example, it has been observed that cancer-related abnormal DNA methylation, one of the important epigenetic marks, on gene promoter observed in parents can be inherited by their children, the question of whether epigenetic signatures are passed on to descendants, especially in mammals, has been a long-standing and fascinating issue of debate. Unlike genomic DNA sequences, which are relatively stable, epigenetic signatures can potentially change in respond to external stimuli, and the transmission of these changes to offspring suggests that the parental generation's growth environment may influence the phenotypes of their descendants. Therefore, determining whether epigenetic information can be transmitted to offspring in mammals, including humans, are crucial for better understanding familial risk of human diseases, such as cancer, heart disease, diabetes, and hypercholesterolemia.
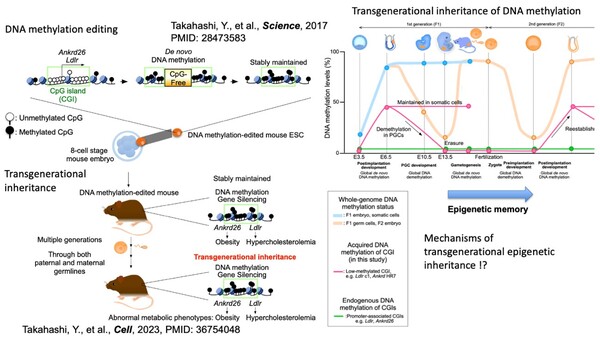
In our previous work, we demonstrated that DNA methylation of gene promoters can be transmitted from parents to their offspring in mice (Y. Takahashi et al., Cell, 2023; 186: 4, 715-731, PMID:36754048). Using our unique DNA methylation editing strategy, which can induce stable DNA methylation at targeted gene promoters (Y. Takahashi et al.,Science, 2017; 356: 503-508, PMID:28473583), we successfully generated the first DNA methylation-edited mice exhibiting the abnormal metabolic phenotypes caused by the edited methylation. Intriguingly, both the acquired methylation and the phenotypic traits were maintained and transmitted across multiple generations. This groundbreaking finding provided the first experimental evidence that epigenetic signatures can be transmitted from parents to their offspring in mammals, as also supported by other scientists' view (Research Highlights: Nature, Nature reviews genetics, Nature genetics, Cell Research,Lab Animal, Cell).
Our laboratory aims to elucidate the mechanisms of transgenerational epigenetic inheritance in mammals using our unique DNA methylation editing technologies, DNA methylation edited mouse models, and human pluripotent stem cell models. These endeavors not only serve to increase our understanding of mammalian embryonic development and evolution but also offer insights into the etiology, diagnosis, and susceptibility of non-genetically inherited human diseases in offspring.
Biography
Yuta Takahashi Ph.D. joined the International Research Center for Medical Sciences (IRCMS) at Kumamoto University as an Associate Professor to launch his own laboratory in April 2024. Throughout Yuta's academic and research journey, his focus has been on exploring the role of epigenetic regulation in various aspects of animal development, tissue physiology, aging, disease progression, and transgenerational transmission. Yuta earned a B.S. in 2006, an M.S. in 2008, and a Ph.D. in 2011 from the University of Tsukuba in Japan, working under the guidance of Dr. Akiyoshi Fukamizu. Notably, as a graduate student, Yuta made a significant discovery in the field by demonstrating that the epigenetic mark arginine methylation regulates the lifespan of the nematode, Caenorhabditis elegans, through the modifications of the forkhead transcription factor (Y. Takahashi et al., Cell Metabolism, 2011). During his postdoctoral training at the Salk Institute, under the mentorship of Dr. Juan Carlos Izpisua Belmonte, Yuta's research focused on elucidating the role of DNA methylation in physiological and pathological states. Yuta's investigations centered on understanding why only CpG-rich regions, known as CpG islands, remain unmethylated in the heavily methylated mammalian genome. Their groundbreaking research revealed that the introduction of CpG-free DNA into targeted CpG islands triggers de novo methylation of CpG islands in human PSCs (Y. Takahashi et al.,Science, 2017). Additionally, as a staff researcher at the Salk Institute, Yuta successfully employed this unique approach to establish the first mouse model with epigenetically modified DNA methylation patterns related to cancer and metabolic disorders (Y. Takahashi et al., Cell, 2023). Moreover, Yuta's groundbreaking research demonstrated the occurrence of transgenerational inheritance of epigenetic signatures and associated phenotypes using the epigenetically modified mouse models (Y. Takahashi et al., Cell, 2023). Yuta then moved to Altos Labs Inc., San Diego Institute of Science, under the leadership of Director Dr. Juan Carlos Izpisua Belmonte in 2022. These findings not only serve as a pioneering contribution to the field of mammalian epigenetic inheritance research but also provide valuable insights into biological evolution and prevention of epigenetic diseases.