- HOME
- News & Events
- Publications
- 【Publications】Phospholipid metabolic adaptation promotes survival of IDH2 mutant acute myeloid leuke...
Publications
【Publications】Phospholipid metabolic adaptation promotes survival of IDH2 mutant acute myeloid leukemia cells
October 30 2023
Lab: Hitoshi Takizawa
Paper information
Tile:
Phospholipid metabolic adaptation promotes survival of IDH2 mutant acute myeloid leukemia cells
Tatsuya Morishima*, Koichi Takahashi, Desmond Wai Loon Chin, Yuxin Wang, Kenji Tokunaga, Yuichiro Arima, Masao Matsuoka, Toshio Suda, Hitoshi Takizawa* (*Corresponding authors)
Cancer Science 2023/10/26 DOI: 10.1111/cas.15994
Highlights
-
Phospholipid metabolic adaptation in IDH2 mutant acute myeloid leukemia (AML) cells underlies their resistance to apoptosis.
-
Food and Drug Administration-approved anti-inflammatory drugs targeting the metabolism of arachidonic acid can sensitize IDH2 mutant AML cells to apoptosis.
Abstract:
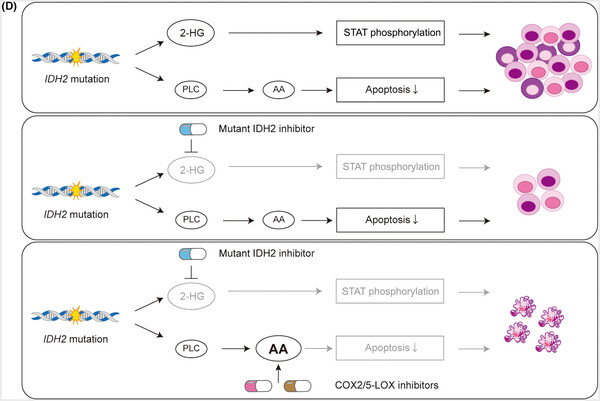
Genetic mutations in the isocitrate dehydrogenase (IDH) gene that result in a pathological enzymatic activity to produce oncometabolite have been detected in acute myeloid leukemia (AML) patients. While specific inhibitors that target mutant IDH enzymes and normalize intracellular oncometabolite level have been developed, refractoriness and resistance has been reported. Since acquisition of pathological enzymatic activity is accompanied by the abrogation of the crucial WT IDH enzymatic activity in IDH mutant cells, aberrant metabolism in IDH mutant cells can potentially persist even after the normalization of intracellular oncometabolite level. Comparisons of isogenic AML cell lines with and without IDH2 gene mutations revealed two mutually exclusive signalings for growth advantage of IDH2 mutant cells, STAT phosphorylation associated with intracellular oncometabolite level and phospholipid metabolic adaptation. The latter came to light after the oncometabolite normalization and increased the resistance of IDH2 mutant cells to arachidonic acid-mediated apoptosis. The release of this metabolic adaptation by FDA-approved anti-inflammatory drugs targeting the metabolism of arachidonic acid could sensitize IDH2 mutant cells to apoptosis, resulting in their eradication in vitro and in vivo. Our findings will contribute to the development of alternative therapeutic options for IDH2 mutant AML patients who do not tolerate currently available therapies.
Graphical abstract