- HOME
- News & Events
- Publications
- 【Publications】Akkermansia muciniphila induces slow extramedullary hematopoiesis via cooperative IL-1...
Publications
【Publications】Akkermansia muciniphila induces slow extramedullary hematopoiesis via cooperative IL-1R/TLR signals
October 27 2023
Lab: Hitoshi Takizawa
Paper information
Tile:
Akkermansia muciniphila induces slow extramedullary hematopoiesis via cooperative IL-1R/TLR signals
Yuxin Wang, Tatsuya Morishima, Maiko Sezaki, Ryo Sato, Gaku Nakato, Shinji Fukuda, Kouji Kobiyama, Ken J Ishii, Yuhua Li, Hitoshi Takizawa
EMBO Reports e57485 2023/10/23 doi: 10.15252/embr.202357485.
Highlights
-
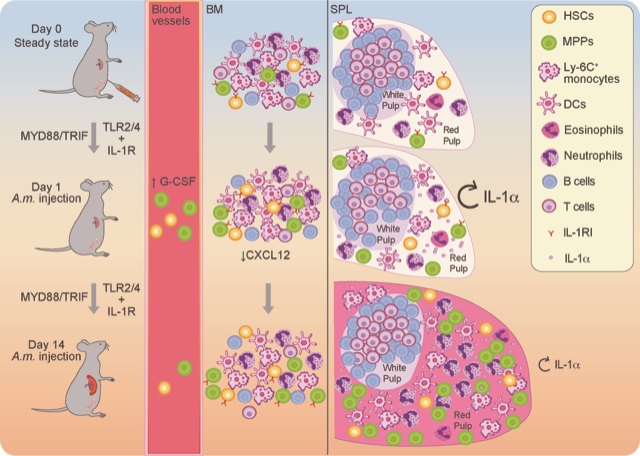
Injection of A. muciniphila lysate induces long-lasting anemia and hepatosplenomegaly with expansion of functional HSCs.
-
Cell expansion in the spleen is mediated by partially TLR2/4-dependent and entirely MYD88/TRIF-dependent pathways.
- Sustained secretion of IL-1α from splenic myeloid cells contributes to TLR2/4-independent EMH.
- Pharmacological inhibition of IL-1R in addition to genetic ablation of TLR2/4 can completely alleviate induced EMH.
Abstract:
Bacterial infections can activate and mobilize hematopoietic stem and progenitor cells (HSPCs) from the bone marrow (BM) to the spleen, a process termed extramedullary hematopoiesis (EMH). Recent studies suggest that commensal bacteria regulate not only the host immune system but also hematopoietic homeostasis. However, the impact of gut microbes on hematopoietic pathology remains unclear. Here, we find that systemic single injections of Akkermansia muciniphila (A. m.), a mucin-degrading bacterium, rapidly activate BM myelopoiesis and slow but long-lasting hepato-splenomegaly, characterized by the expansion and differentiation of functional HSPCs, which we term delayed EMH. Mechanistically, delayed EMH triggered by A. m. is mediated entirely by the MYD88/TRIF innate immune signaling pathway, which persistently stimulates splenic myeloid cells to secrete interleukin (IL)-1α, and in turn, activates IL-1 receptor (IL-1R)-expressing splenic HSPCs. Genetic deletion of Toll-like receptor-2 and -4 (TLR2/4) or IL-1α partially diminishes A. m.-induced delayed EMH, while inhibition of both pathways alleviates splenomegaly and EMH. Our results demonstrate that cooperative IL-1R- and TLR-mediated signals regulate commensal bacteria-driven EMH, which might be relevant for certain autoimmune disorders.
Graphical abstract