- HOME
- News & Events
- Publications
- 【Publications】Unique molecular and functional features of extramedullary hematopoietic stem and prog...
Publications
【Publications】Unique molecular and functional features of extramedullary hematopoietic stem and progenitor cell reservoirs in humans
January 27 2022
Lab: Hitoshi Takizawa
Paper information
Title:
Unique molecular and functional features of extramedullary hematopoietic stem and progenitor cell reservoirs in humans
Mende N, Bastos HP, Santoro A, Mahbubani KT, Ciaurro V, Calderbank EF, Quiroga Londoño M, Sham K, Mantica G, Morishima T, Mitchell E, Lidonnici MR, Meier-Abt F, Hayler D, Jardine L, Curd A, Haniffa M, Ferrari G, Takizawa H, Wilson NK, Gottgens B, Saeb-Parsy K, Frontini M, Laurenti E.
Blood. January 24, 2022
Highlights
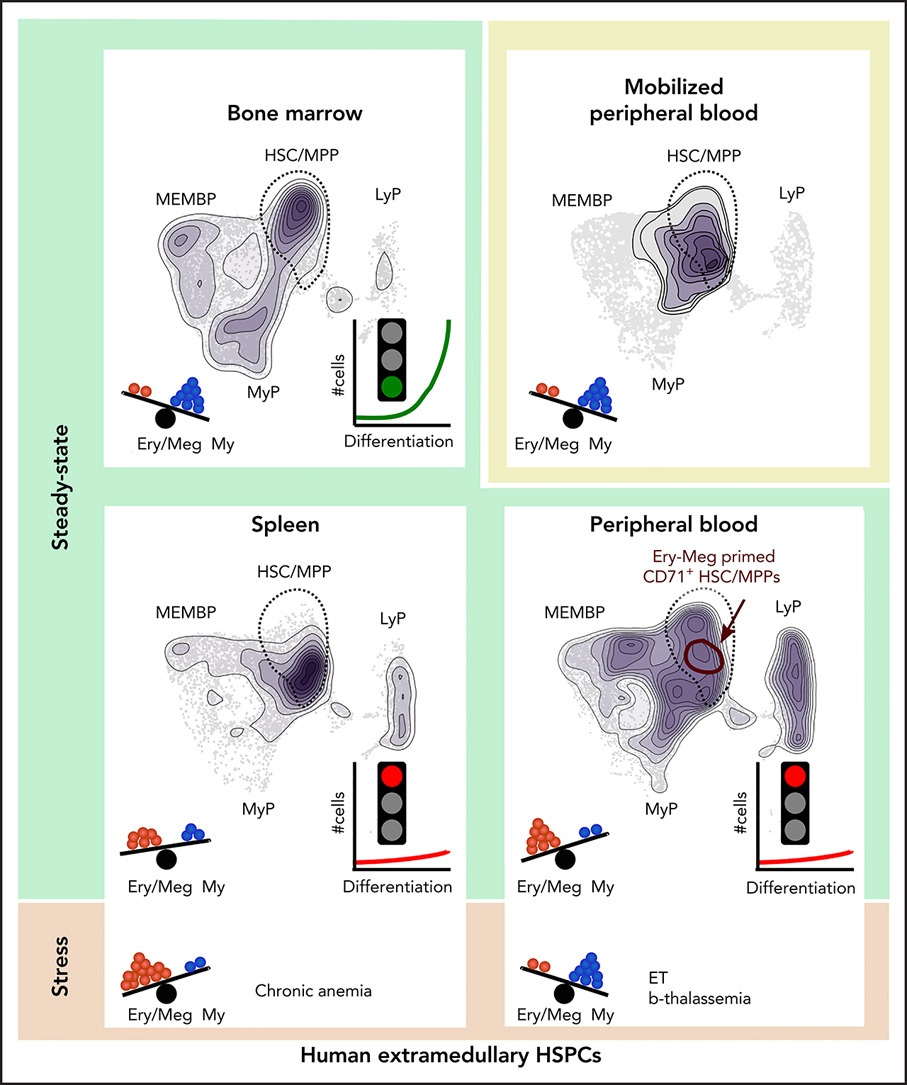
- Extramedullary tissues harbor reservoirs of HSC/MPPs and non-proliferative progenitors without ongoing hematopoiesis.
- Functional bias of peripheral blood HSPCs towards erythropoiesis, mediated by CD71+ HSC/MPPs and suppressed with age and disease.
Abstract
Rare hematopoietic stem and progenitor cell (HSPC) pools outside the bone marrow (BM) contribute to blood production in stress and disease but remain ill-defined. Although non-mobilized peripheral blood (PB) is routinely sampled for clinical management, the diagnosis and monitoring potential of PB HSPCs remains untapped, as no healthy PB HSPC baseline has been reported. Here we comprehensively delineate human extramedullary HSPC compartments comparing spleen, PB and mobilized PB (mPB) to BM using single-cell RNA-seq and/or functional assays. We uncover HSPC features shared by extramedullary tissues and others unique to PB. First, in contrast to actively dividing BM HSPCs, we find no evidence of substantial ongoing hematopoiesis in extramedullary tissues at steady state, but report increased splenic HSPC proliferative output during stress erythropoiesis. Second, extramedullary stem cells/multipotent progenitors (HSC/MPPs) from spleen, PB and mPB share a common transcriptional signature and increased abundance of lineage-primed subsets compared to BM. Third, healthy PB HSPCs display a unique bias towards erythroid-megakaryocytic differentiation. At HSC/MPP level, this is functionally imparted by a subset of phenotypic CD71+ HSC/MPPs, exclusively producing erythrocytes and megakaryocytes, highly abundant in PB but rare in other adult tissues. Finally, the unique erythroid-megakaryocytic-skewing of PB is perturbed with age, in essential thrombocythemia and in beta-thalassemia. Collectively, we identify extramedullary lineage-primed HSPC reservoirs that are non-proliferative in situ and report involvement of splenic HSPCs during demand-adapted hematopoiesis. Our data also establish aberrant composition and function of circulating HSPCs as potential clinical indicators of BM dysfunction.