- HOME
- News & Events
- Publications
- 【Publications】IL-1 Mediates Microbiome-Induced Inflamm-Ageing of Hematopoietic Stem Cells in Mice
Publications
【Publications】IL-1 Mediates Microbiome-Induced Inflamm-Ageing of Hematopoietic Stem Cells in Mice
September 22 2021
Lab: Hitoshi Takizawa
Paper Information
Title:
IL-1 Mediates Microbiome-Induced Inflamm-Ageing of Hematopoietic Stem Cells in Mice
Larisa Vladimirovna Kovtonyuk, Francisco Caiado, Santiago Garcia-Martin, Eva-Maria Manz, Patrick Michael Helbling, Hitoshi Takizawa, Steffen Boettcher, Fatima AI-Shahror, Cesar Nombela-Arrieta, Emma Slack, Markus G Manz
Blood.
Online Ahead of Editing April 30th, 2021
doi: https://doi.org/10.1182/blood.2021011570
Highlights
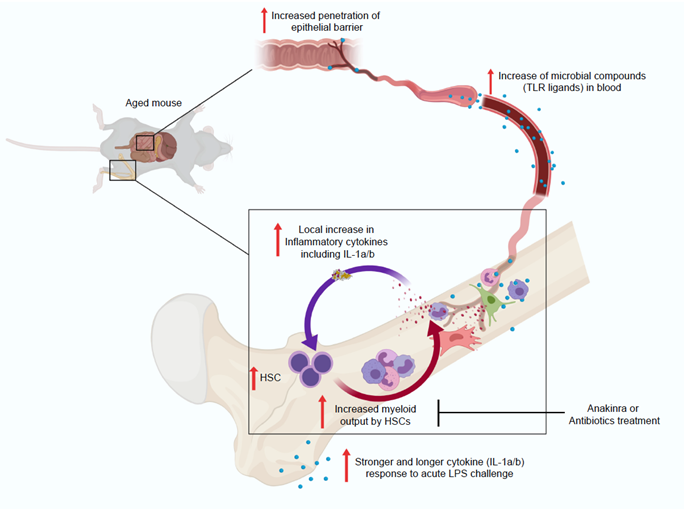
- Increased blood microbial compounds drive elevated IL-1a/b production in bone marrow of ageing mice
- HSC in aged IL-1R1KO and in germ-free mice are protected from, and IL-1 antagonist or antibiotic treatment revert HSC inflamm-ageing
Abstract
Ageing is associated with impaired hematopoietic and immune function. This is caused in part by decreased hematopoietic stem cell (HSC) population fitness and an increased myeloid differentiation bias. The reasons for this aging-associated HSC impairment are incompletely understood. We here demonstrate that aged specific pathogen free (SPF) wild-type mice in contrast to young SPF mice produce more IL-1a/b in steady-state bone marrow (BM), with most of IL-1a/b being derived from myeloid BM cells. Further, blood of steady-state aged SPF wild-type mice contains higher levels of microbe associated molecular patterns (MAMPs), specifically TLR4 and TLR8 ligands. Also, BM myeloid cells from aged mice produce more IL-1b in vitro, and aged mice show higher and more durable IL-1a/b responses upon LPS stimulation in vivo. To test if HSC ageing is driven via IL-1a/b, we evaluated HSCs from IL-1 receptor 1 (IL-1R1) knock-out mice. Indeed, aged HSCs from IL-1R1 knock-out mice show significantly mitigated ageing-associated inflammatory signatures. Moreover, HSCs from aged IL-1R1KO and also from germ-free mice maintain unbiased lympho-myeloid hematopoietic differentiation upon transplantation, thus resembling this functionality of young HSCs. Importantly, in vivo antibiotic suppression of microbiota or pharmacologic blockade of IL-1 signaling in aged wild-type mice was similarly sufficient to reverse myeloid biased output of their HSC populations. Collectively, our data defines the microbiome-IL-1/IL-1R1 axis as a key, self-sustaining, but also therapeutically partially reversible driver of HSC inflamm-ageing.