- HOME
- News & Events
- Publications
- 【Publications】Metabolic shift to serine biosynthesis through 3-PG accumulation and PHGDH induction p...
Publications
【Publications】Metabolic shift to serine biosynthesis through 3-PG accumulation and PHGDH induction promotes tumor growth in pancreatic cancer
September 24 2021
Lab: Takatsugu Ishimoto
Paper information
Title:
Metabolic shift to serine biosynthesis through 3-PG accumulation and PHGDH induction promotes tumor growth in pancreatic cancer
Itoyama R, Yasuda-Yoshihara N, Kitamura F, Yasuda T, Bu L, Yonemura A, Uchihara T, Arima K, Hu X, Jun Z, Okamoto Y, Akiyama T, Yamashita K, Nakao Y, Yusa T, Kitano Y, Higashi T, Miyata T, Imai K, Hayashi H, Yamashita YI, Mikawa T, Kondoh H, Baba H*, Ishimoto T*.
(*corresponding authors)
Cancer Letters 2021 Sep 8:S0304-3835(21)00454-7.
doi:10.1016/j.canlet.2021.09.007.
Highlights
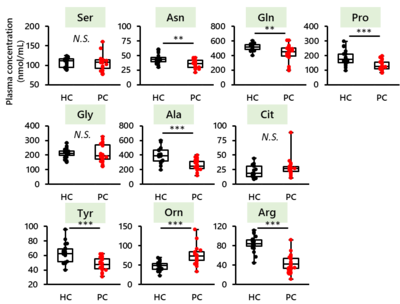
- High PHGDH expression is significantly correlated with a high serum serine concentration and poor prognosis in PDAC patients.
- DNA hypomethylation in CpG islands of the PHGDH gene locus has a strong impact on PHGDH induction in PDAC cells under serine deprivation.
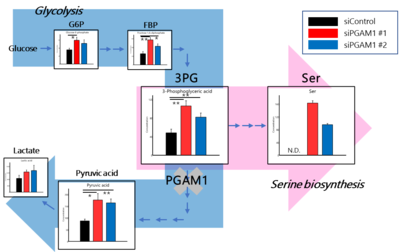
- 3-PG accumulation via PGAM1 downregulation enhances serine biosynthesis and tumor growth under serine deprivation.
- PHGDH inhibition shows a significant suppressive effect on tumor growth under serine deprivation.
Abstract
Cancer cells craftily adapt their energy metabolism to their microenvironment. Nutrient deprivation due to hypovascularity and fibrosis is a major characteristic of pancreatic ductal adenocarcinoma (PDAC); thus, PDAC cells must produce energy intrinsically. However, the enhancement of energy production via activating Kras mutations is insufficient to explain the metabolic rewiring of PDAC cells. Here, we investigated the molecular mechanism underlying the metabolic shift in PDAC cells under serine starvation. Amino acid analysis revealed that the concentrations of all essential amino acids and most nonessential amino acids were decreased in the blood of PDAC patients. In addition, the plasma serine concentration was significantly higher in PDAC patients with PHGDH-high tumors than in those with PHGDH-low tumors. Although the growth and tumorigenesis of PK-59 cells with PHGDH promoter hypermethylation were significantly decreased by serine starvation, these activities were maintained in PDAC cell lines with PHGDH promoter hypomethylation by serine biosynthesis through PHGDH induction. In fact, DNA methylation analysis by pyrosequencing revealed that the methylation status of the PHGDH promoter was inversely correlated with the PHGDH expression level in human PDAC tissues. In addition to PHGDH induction by serine starvation, PDAC cells showed enhanced serine biosynthesis under serine starvation through 3-PG accumulation via PGAM1 knockdown, resulting in enhanced PDAC cell growth and tumor growth. However, PHGDH knockdown efficiently suppressed PDAC cell growth and tumor growth under serine starvation. These findings provide evidence that targeting the serine biosynthesis pathway by inhibiting PHGDH is a potent therapeutic approach to eliminate PDAC cells in nutrient-deprived microenvironments.
Representative Figure