- HOME
- News & Events
- Publications
- 【Publications】Numerical evaluation reveals the effect of branching morphology on vessel transport pr...
Publications
【Publications】Numerical evaluation reveals the effect of branching morphology on vessel transport properties during angiogenesis
June 21 2021
Masanori Nakayama (IRCMS Visiting Associate Professor)
Paper information
Title:
Numerical evaluation reveals the effect of branching morphology on vessel transport properties during angiogenesis
Fatemeh Mirzapour-Shafiyi, Yukinori Kametani, Takao Hikita, Yosuke Hasegawa, Masanori Nakayama
Plos Computational Biology. June 16, 2021
doi:10.1371/journal.pcbi.1008398
Highlights
1. Various factors which regulate blood vessel branching morphology have been identified, however, their impacts on the vascular transport properties of oxygen remain elusive.
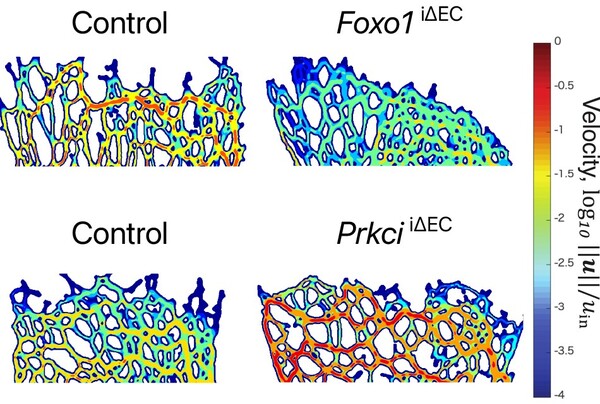
2. We reconstruct 3D vessel structures from 2D confocal microscopy images, then numerically simulate blood flow in the structures.
3. Vessel network complexity negatively affects the blood perfusion efficiency and tissue oxygenation during angiogenesis.
Abstract
Blood flow governs transport of oxygen and nutrients into tissues. Hypoxic tissues secrete VEGFs to promote angiogenesis during development and in tissue homeostasis. In contrast, tumors enhance pathologic angiogenesis during growth and metastasis, suggesting suppression of tumor angiogenesis could limit tumor growth. In line with these observations, various factors have been identified to control vessel formation in the last decades. However, their impact on the vascular transport properties of oxygen remain elusive.Here, we take a computational approach to examine the effects of vascular branching on blood flow in the growing vasculature. First of all, we reconstruct a 3D vascular model from the 2D confocal images of the growing vasculature at postnatal day 5 (P5) mouse retina, then simulate blood flow in the vasculatures, which are obtained from the gene targeting mouse models causing hypo- or hyper-branching vascular formation. Interestingly, hyper-branching morphology attenuates effective blood flow at the angiogenic front, likely promoting tissue hypoxia. In contrast, vascular hypo-branching enhances blood supply at the angiogenic front of the growing vasculature. Oxygen supply by newly formed blood vessels improves local hypoxia and decreases VEGF expression at the angiogenic front during angiogenesis. Consistent with the simulation results indicating improved blood flow in the hypo-branching vasculature, VEGF expression around the angiogenic front is reduced in those mouse retinas. Conversely, VEGF expression is enhanced in the angiogenic front of hyper-branching vasculature. Our results indicate the importance of detailed flow analysis in evaluating the vascular transport properties of branching morphology of the blood vessels.
(Click for a larger image)