- HOME
- News & Events
- Publications
- 【Publications】Murine neonatal ketogenesis preserves mitochondrial energetics by preventing protein h...
Publications
【Publications】Murine neonatal ketogenesis preserves mitochondrial energetics by preventing protein hyperacetylation
February 19 2021
Yuichiro Arima
Paper information
Yuichiro Arima*, Yoshiko Nakagawa*, Toru Takeo*, Toshifumi Ishida, Toshihiro Yamada, Shinjiro Hino, Mitsuyoshi Nakao, Sanshiro Hanada, Terumasa Umemoto, Toshio Suda, Tetsushi Sakuma, Takashi Yamamoto, Takehisa Watanabe, Katsuya Nagaoka, Yasuhito Tanaka, Yumiko K Kawamura, Kazuo Tonami, Hiroki Kurihara, Yoshifumi Sato, Kazuya Yamagata, Taishi Nakamura, Satoshi Araki, Eiichiro Yamamoto, Yasuhiro Izumiya, Kenji Sakamoto, Koichi Kaikita, Kenichi Matsushita, Koichi Nishiyama, Naomi Nakagata, and Kenichi Tsujita
(*These authors equally contributed to this work.)
Title
Murine neonatal ketogenesis preserves mitochondrial energetics by preventing protein hyperacetylation
Nature Metabolism volume 3, pages196-210(2021)
doi: 10.1038/s42255-021-00342-6
URL: https://www.nature.com/articles/s42255-021-00342-6
Highlights
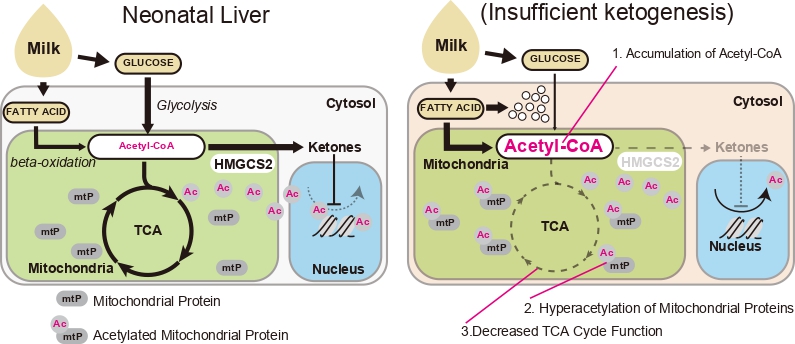
Arima et al. find the novel function of ketone body metabolism. They determine that hepatic ketogenesis prevents hyperacetlyation of mitochondrial proteins and disruption of mitochondrial energetics.
Abstract
Ketone bodies are generated in the liver and allow for the maintenance of systemic caloric and energy homeostasis during fasting and caloric restriction. It has previously been demonstrated that neonatal ketogenesis is activated independently of starvation. However, the role of ketogenesis during the perinatal period remains unclear. Here, we show that neonatal ketogenesis plays a protective role in mitochondrial function. We generated a murine model of insufficient ketogenesis by disrupting the rate-limiting hydroxymethylglutaryl (HMG)-CoA synthase 2 enzyme gene (Hmgcs2). Hmgcs2 knockout (KO) neonates develop microvesicular steatosis within a few days of birth. Electron microscopic analysis and metabolite profiling indicate a restricted energy production capacity and accumulation of acetyl-CoA in Hmgcs2 KO mice. Furthermore, acetylome analysis of Hmgcs2 KO cells reveals enhanced acetylation of mitochondrial proteins. These findings suggest that neonatal ketogenesis protects the energy-producing capacity of mitochondria by preventing the hyperacetylation of mitochondrial proteins.