- HOME
- News & Events
- Publications
- 【Publications】A FLCN-TFE3 feedback loop prevents excessive glycogenesis and phagocyte activation by ...
Publications
【Publications】A FLCN-TFE3 feedback loop prevents excessive glycogenesis and phagocyte activation by regulating lysosome activity
February 11 2020
Toshio Suda, Masaya Baba
Mitsuhiro Endoh#*, Masaya Baba#*, Tamie Endoh, Akiyoshi Hirayama, Ayako Nakamura-Ishizu, Terumasa Umemoto, Michihiro Hashimoto, Kunio Nagashima, Tomoyoshi Soga, Martin Lang, Laura S. Schmidt, W. Marston Linehan, Toshio Suda*
(#These authors contributed equally to this work, *corresponding authors)
Cell Reports 2020 Feb 11. doi: https://www.sciencedirect.com/science/article/pii/S2211124720300668?via%3Dihub
Highlights
- Flcn knockout in mice induces phagocytes accumulating cytoplasmic glycogen
- Tfe3 deletion in Flcn knockout mice ameliorates the phenotypes
- Loss of Flcn induces chronic activation of Tfe3 and lysosomal function
- Tfe3 promotes glycogenesis gene expression upon Flcn loss
Abstract
The tumor suppressor folliculin (FLCN) suppresses nuclear translocation of TFE3, a master transcription factor for lysosomal biogenesis, via regulation of amino-acid-sensing Rag GTPases. However, the importance of this lysosomal regulation in mammalian physiology remains unclear. Following hematopoietic-lineage-specific Flcn deletion in mice, we found expansion of vacuolated phagocytes that accumulate glycogen in their cytoplasm, phenotypes reminiscent of lysosomal storage disorder (LSD). We report that TFE3 acts in a feedback loop to transcriptionally activate FLCN expression, and FLCN loss disrupts this loop, augmenting TFE3 activity. Tfe3 deletion in Flcn knockout mice reduces the number of phagocytes and ameliorates LSD-like phenotypes. We further reveal that TFE3 stimulates glycogenesis by promoting the expression of glycogenesis genes, including Gys1 and Gyg, upon loss of Flcn. Taken together, we propose that the FLCN-TFE3 feedback loop acts as a rheostat to control lysosome activity and prevents excessive glycogenesis and LSD-like phagocyte activation.
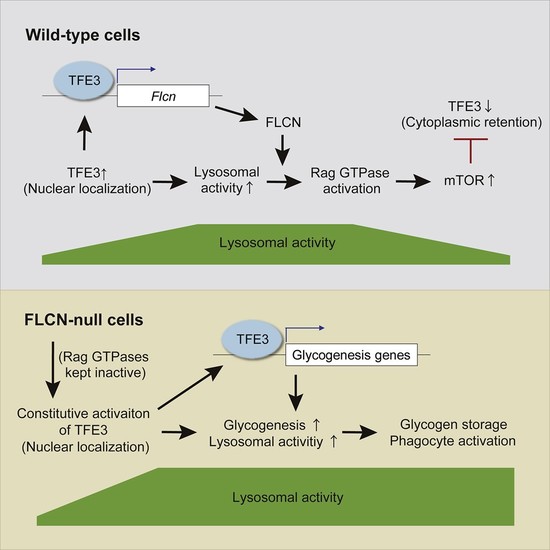
Hematopoietic lineage-specific Flcn deletion in mice induces expansion of phagocytes accumulating cytoplasmic glycogen. Tfe3 acts in a feedback loop to activate Flcn expression, and Flcn loss disrupts this loop, augmenting Tfe3 activity. Tfe3 deletion in Flcn knockout mice blocks aberrant glycogenesis and ameliorates the phenotypes. TFE3 stimulates glycogenesis by promoting the expression of glycogenesis genes, including Gys1 and Gyg, upon loss of Flcn.
