- HOME
- News & Events
- Publications
- 【Publications】Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of ...
Publications
【Publications】Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid
January 7 2020
Koichi Nishiyama, Yuichiro Arima
Paper information
Yuji Nashimoto1, 2, 3, Ryu Okada1, Sanshiro Hanada4 Yuichiro Arima4, Koichi Nishiyama4, Takashi Miura5, Ryuji Yokokawa1, * (* corresponding author)
1 Department of Micro Engineering, Kyoto University, Kyoto, 615-8540, Japan
2 Frontier Research Institute for Interdisciplinary Sciences (FRIS), Tohoku University, Miyagi, 980-8578, Japan
3 Graduate School of Engineering, Tohoku University, Miyagi, 980-8579, Japan
4 International Research Center for Medical Sciences (IRCMS), Kumamoto University, Kumamoto, 860-0811, Japan
5 Graduate School of Medical Sciences, Kyushu University, Fukuoka, 812-8582, Japan
Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid
Biomaterials. 2020 Jan;229:119547. doi: 10.1016/j.biomaterials.2019.119547.
Highlights
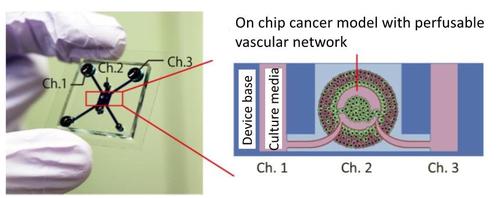
- Establishment of a tumor-on-a-chip platform with perfusable vascular network
- The platform is applicable to a drug screening platform as an alternative to conventional animal models
Abstract
Tumor vasculature creates a hostile tumor microenvironment (TME) in vivo and nourishes cancers, resulting in cancer progression and drug resistance. To mimic the biochemical and biomechanical environments of tumors in vitro, several models integrated with a vascular network have been reported. However, the tumor responses to biochemical and biomechanical stimuli were evaluated under static conditions and failed to incorporate the effects of blood flow to tumors. In this study, we present a tumor-on-a-chip platform that enables the evaluation of tumor activities with intraluminal flow in an engineered tumor vascular network. The fibroblasts in the tumor spheroid induced angiogenic sprouts, which constructed a perfusable vascular network in a tumor spheroid. The perfusability of the engineered vascular network was preserved during the culture. Moreover, perfusion for over 24 h significantly increased the proliferation activities of tumor cells and decreased cell death in the spheroid. Drug administration under perfusion condition did not show the dose-dependent effects of anticancer drugs on tumor activities in contrast to the results under static conditions. Our results demonstrate the importance of flow in a vascular network for the evaluation of tumor activities in a drug screening platform.