- HOME
- News & Events
- Publications
- 【Publications】LMO2 activation by deacetylation is indispensable for hematopoiesis and T-ALL leukemog...
Publications
【Publications】LMO2 activation by deacetylation is indispensable for hematopoiesis and T-ALL leukemogenesis
August 22 2019
Tatsuya Morishima
Paper information
Tatsuya Morishima*, Ann-Christin Krahl, Masoud Nasri, Yun Xu, Narges Aghaallaei, Betül Findik, Maksim Klimiankou, Malte Ritter, Marcus D. Hartmann, Christian Johannes Gloeckner, Sylwia Stefanczyk, Christian Lindner, Benedikt Oswald, Regine Bernhard, Karin Hähnel, Ursula Hermanutz-Klein, Martin Ebinger, Rupert Handgretinger, Nicolas Casadei, Karl Welte, Maya Andre, Patrick Müller, Baubak Bajoghli, Julia Skokowa* (*:Corresponding authors)
LMO2 activation by deacetylation is indispensable for hematopoiesis and T-ALL leukemogenesis
Blood. 2019 Jul 31. pii: blood.2019000095. doi: 10.1182/blood.2019000095.
Highlights
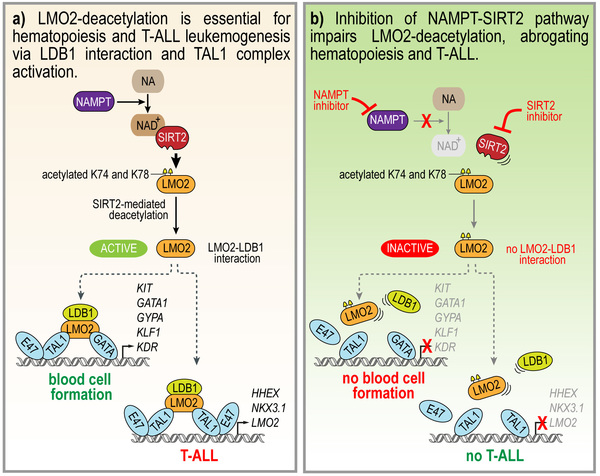
- LMO2 is deacetylated by the NAMPT/SIRT2 pathway.
- LMO2 deacetylation is essential for LDB1 binding and TAL1 complex activation during hematopoiesis and T-ALL leukemogenesis.
Abstract
LMO2 (hematopoietic transcription factor LIM domain only 2), a member of the TAL1 transcriptional complex, plays an essential role during early hematopoiesis and is frequently activated in T cell acute lymphoblastic leukemia (T-ALL) patients. Here, we demonstrated that LMO2 is activated by deacetylation on lysine 74 and 78 via the nicotinamide phosphoribosyltransferase (NAMPT)/sirtuin 2 (SIRT2) pathway. LMO2 deacetylation enables LMO2 to interact with LDB1 and activate the TAL1 complex. NAMPT/SIRT2-mediated activation of LMO2 by deacetylation is essential for hematopoietic differentiation of induced pluripotent stem (iPS) cells and blood formation in zebrafish embryos. In T-ALL, deacetylated LMO2 induces expression of TAL1 complex target genes HHEX, NKX3.1 as well as LMO2 autoregulation. Consistent with this, inhibition of NAMPT or SIRT2 suppressed the in vitro growth and in vivo engraftment of T-ALL cells via diminished LMO2 deacetylation. This new molecular mechanism may provide new therapeutic possibilities in T-ALL and may contribute to the development of new methods for in vitro generation of blood cells.