- HOME
- News & Events
- Publications
- 【Publications】TFE3 Xp11.2 translocation renal cell carcinoma mouse model reveals novel therapeutic t...
Publications
【Publications】TFE3 Xp11.2 translocation renal cell carcinoma mouse model reveals novel therapeutic targets and identifies GPNMB as a diagnostic marker for human disease.
May 15 2019
Masaya Baba
Paper information
Masaya Baba, Mitsuko Furuya, Takanobu Motoshima, Martin Lang, Shintaro Funasaki, Wenjuan Ma, Hong-Wei Sun, Hisashi Hasumi, Ying Huang, Ikuma Kato, Tsuyoshi Kadomatsu, Yorifumi Satou, Nicole Morris, Baktiar O Karim, Lilia Ileva, Joseph D Kalen, Luh Ade Wilan Krisna, Yukiko Hasumi, Aiko Sugiyama, Ryoma Kurahashi, Koshiro Nishimoto, Masafumi Oyama, Yoji Nagashima, Naoto Kuroda, Kimi Araki, Masatoshi Eto, Masahiro Yao, Tomomi Kamba, Toshio Suda, Yuichi Oike, Laura S Schmidt, W Marston Linehan
TFE3 Xp11.2 translocation renal cell carcinoma mouse model reveals novel therapeutic targets and identifies GPNMB as a diagnostic marker for human disease
Mol Can Res. 2019 May 1. doi: 10.1158/1541-7786.MCR-18-1235. [Epub ahead of print]
Highlights
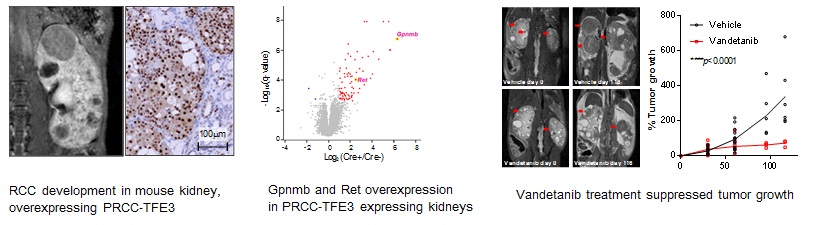
- Xp11.2 translocation renal cell carcinoma (TFE3-RCC) is highly aggressive with poor prognosis and no established therapies for advanced cases.
- We generated a mouse model with kidney-specific PRCC-TFE3 fusion transgene expression that developed multiple kidney cysts and tumors.
- Expression of the receptor tyrosine kinase Ret was elevated in kidneys of PRCC-TFE3 mice and treatment with Ret inhibitor vandetanib suppressed tumor growth, supporting RET as a potential therapeutic target.
- Glycoprotein nonmetastatic B (Gpnmb) was shown to be a biomarker for human and mouse TFE3-RCC, establishing its role as diagnostic marker for TFE3-RCC.
Abstract
Renal Cell Carcinoma (RCC) associated with Xp11.2 translocation (TFE3-RCC) has been recently defined as a distinct subset of RCC classified by characteristic morphology and clinical presentation. The Xp11 translocations involve the TFE3 transcription factor and produce chimeric TFE3 proteins retaining the basic helix-loop-helix leucine zipper structure for dimerization and DNA binding suggesting that chimeric TFE3 proteins function as oncogenic transcription factors. Diagnostic biomarkers and effective forms of therapy for advanced cases of TFE3-RCC are as yet unavailable. To facilitate the development of molecular-based diagnostic tools and targeted therapies for this aggressive kidney cancer, we generated a translocation RCC mouse model, in which the PRCC-TFE3 transgene is expressed specifically in kidneys leading to the development of RCC with characteristic histology. Expression of the receptor tyrosine kinase Ret was elevated in the kidneys of the TFE3-RCC mice, and treatment with RET inhibitor vandetanib significantly suppressed RCC growth. Moreover, we found that Gpnmb (Glycoprotein nonmetastatic B) expression was notably elevated in the TFE3-RCC mouse kidneys as seen in human TFE3-RCC tumors, and confirmed that GPNMB is the direct transcriptional target of TFE3 fusions. While GPNMB immunohistochemical staining was positive in 9/9 cases of TFE3-RCC, Cathepsin K, a conventional marker for TFE3-RCC, was positive in only 67% of cases. These data support RET as a potential target and GPNMB as a diagnostic marker for TFE3-RCC. The TFE3-RCC mouse provides a preclinical in vivo model for the development of new biomarkers and targeted therapeutics for patients affected with this aggressive form of renal cell carcinoma.
Implications:
Key findings from studies with this preclinical mouse model of TFE-RCC underscore the potential for RET as a therapeutic target for treatment of patients with TFE3-RCC, and suggest that GPNMB may serve as diagnostic biomarker for TFE3 fusion renal cell carcinoma.