- HOME
- News & Events
- Publications
- 【Publications】Inhibition of 15-PGDH causes Kras-driven tumor expansion through prostaglandin E2-ALDH...
Publications
【Publications】Inhibition of 15-PGDH causes Kras-driven tumor expansion through prostaglandin E2-ALDH1 signaling in the pancreas.
November 22 2018
Takatsugu Ishimoto
Paper information
Kota Arima, Masaki Ohmuraya, Keisuke Miyake, Mayu Koiwa, Tomoyuki Uchihara, Daisuke Izumi, Feng Gao, Atsuko Yonemura, Luke Bu, Hirohisa Okabe, Katsunori Imai, Daisuke Hashimoto, Yoshifumi Baba, Akira Chikamoto, Yo-ichi Yamashita, Toru Furukawa, Kimi Araki, Hideo Baba* and Takatsugu Ishimoto*
(*corresponding authors)
Inhibition of 15-PGDH causes Kras-driven tumor expansion through prostaglandin E2-ALDH1 signaling in the pancreas.
Oncogene. 2018 Sep 24. doi: 10.1038/s41388-018-0510-y. [Epub ahead of print]
Highlights
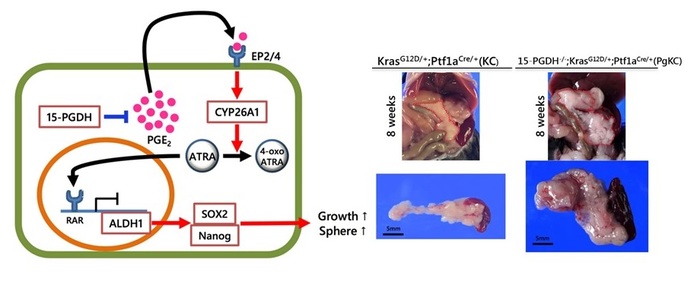
- 15-PGDH is down-regulated in human PDAC and inversely correlated with ALDH1 levels
- 15-PGDH inhibition promotes ALDH1-positive tumor cells and enhances tumor progression via ATRA depletion
- Genetic deletion of 15-Pgdh causes PDAC in mice harboring KrasG12
- ATRA replacement suppresses ALDH1 signaling and pancreatic tumor progression
Abstract
The accumulation of prostaglandin E2 (PGE2) during chronic inflammation has been implicated in the progression of several cancers. Cyclooxygenase is the key enzyme of PGE2, although the degradation enzyme 15-hydroxyprostaglandin dehydrogenase (15-PGDH) has received considerable attention recently. We investigated the molecular mechanisms of pancreatic ductal adenocarcinoma (PDAC) progression via 15-PGDH downregulation. Here, we found that 15-PGDH expression was inversely correlated with ALDH1, an important cancer stem cell-associated marker indicative of poor prognosis in humans. Moreover, we demonstrated that pharmacological inhibition of 15-PGDH enhanced CYP26A1 expression, leading to depletion of all-trans retinoic acid (ATRA) and expansion of the ALDH1-positive subset in both human PDAC cells and tumor cells of KrasLSL-G12D/+; Ptf1aCre/+ (KC) mice. Furthermore, genetic deletion of 15-Pgdh in KC mice showed PGE2 accumulation and ATRA depletion in the pancreas, resulting in PDAC with high levels of Aldh1 and Ki-67. Finally, ATRA replacement suppressed 15-PGDH inhibition-induced tumor progression in KC mice, and ATRA treatment attenuated Aldh1 activity in tumor cells isolated from the pancreas of 15-Pgdh-/- KC mice. These findings provide evidence that 15-PGDH inhibition enhances KRAS-driven tumor progression via ATRA depletion in the pancreas. Therefore, ATRA replacement could be a potential strategy for PDAC treatment.