- HOME
- News & Events
- Publications
- 【Publications】Folliculin regulates osteoclastogenesis through metabolic regulation.
Publications
【Publications】Folliculin regulates osteoclastogenesis through metabolic regulation.
July 19 2018
Masaya Baba
Paper information
Masaya Baba#,*, Mitsuhiro Endoh#, Wenjuan Ma#, Hirofumi Toyama, Akiyoshi Hirayama, Keizo Nishikawa, Keiyo Takubo, Hiroyuki Hano, Hisashi Hasumi, Terumasa Umemoto, Michihiro Hashimoto, Nobuko Irie, Chiharu Esumi, Miho Kataoka, Naomi Nakagata, Tomoyoshi Soga, Masahiro Yao, Tomomi Kamba, Takashi Minami, Masaru Ishii, and Toshio Suda*
(#These authors contributed equally to this work, *corresponding authors)
Folliculin regulates osteoclastogenesis through metabolic regulation.
J Bone Miner Res. 2018 Jun 12. doi: 10.1002/jbmr.3477. [Epub ahead of print]
Highlights
- Osteoclast differentiation (osteoclastogenesis) is a dynamic differentiation process, accompanied by metabolic reprogramming and dynamic gene expression changes.
- Folliculin (Flcn) deficient osteoclast precursors reveal cell autonomous accelerated osteoclastogenesis.
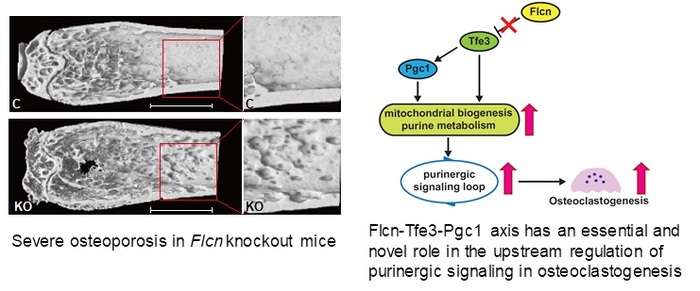
- Flcn regulates oxidative phosphorylation and purine metabolism through suppression of nuclear localization of the transcription factor Tfe3 thereby inhibiting expression of its target gene Pgc1.
- Flcn deficient osteoclast precursors exhibit significant augmentation of oxidative phosphorylation and nucleotide production, resulting in an enhanced purinergic signaling loop
- Inhibition of the purinergic signaling loop efficiently blocks accelerated osteoclastogenesis in Flcn deficient osteoclast precursors.
Abstract
Osteoclast differentiation is a dynamic differentiation process, which is accompanied by dramatic changes in metabolic status as well as in gene expression. Recent findings have revealed an essential connection between metabolic reprogramming and dynamic gene expression changes during osteoclast differentiation. However, the upstream regulatory mechanisms that drive these metabolic changes in osteoclastogenesis remain to be elucidated. Here we demonstrate that induced deletion of a tumor suppressor gene, Folliculin (Flcn), in mouse osteoclast precursors causes severe osteoporosis in 3 weeks through excess osteoclastogenesis. Flcn deficient osteoclast precursors reveal cell autonomous accelerated osteoclastogenesis with increased sensitivity to receptor activator of nuclear factor κB ligand (RANKL). We demonstrate that Flcn regulates oxidative phosphorylation and purine metabolism through suppression of nuclear localization of the transcription factor Tfe3 thereby inhibiting expression of its target gene Pgc1. Metabolome studies revealed that Flcn deficient osteoclast precursors exhibit significant augmentation of oxidative phosphorylation and nucleotide production, resulting in an enhanced purinergic signaling loop which is composed of controlled ATP release and autocrine/paracrine purinergic receptor stimulation. Inhibition of this purinergic signaling loop efficiently blocks accelerated osteoclastogenesis in Flcn deficient osteoclast precursors. Here, we demonstrate an essential and novel role of the Flcn-Tfe3-Pgc1 axis in osteoclastogenesis through the metabolic reprograming of oxidative phosphorylation and purine metabolism.