- HOME
- News & Events
- Publications
- 【Publications】Novel conserved-region T-cell mosaic vaccine with high global HIV-1 coverage is recogn...
Publications
【Publications】Novel conserved-region T-cell mosaic vaccine with high global HIV-1 coverage is recognized by protective responses in untreated infection
May 12 2016
Tomas Hanke
Paper information
Ondondo B, Murakoshi H, Clutton G, Abdul-Jawad S, Wee E G-T, Gatanaga H, Oka S, McMichael AJ., Takiguchi M, Korber B and Hanke T
Novel conserved-region T-cell mosaic vaccine with high global HIV-1 coverage is recognized by protective responses in untreated infection, Mol Ther 24:832-842, 2016
Highlights
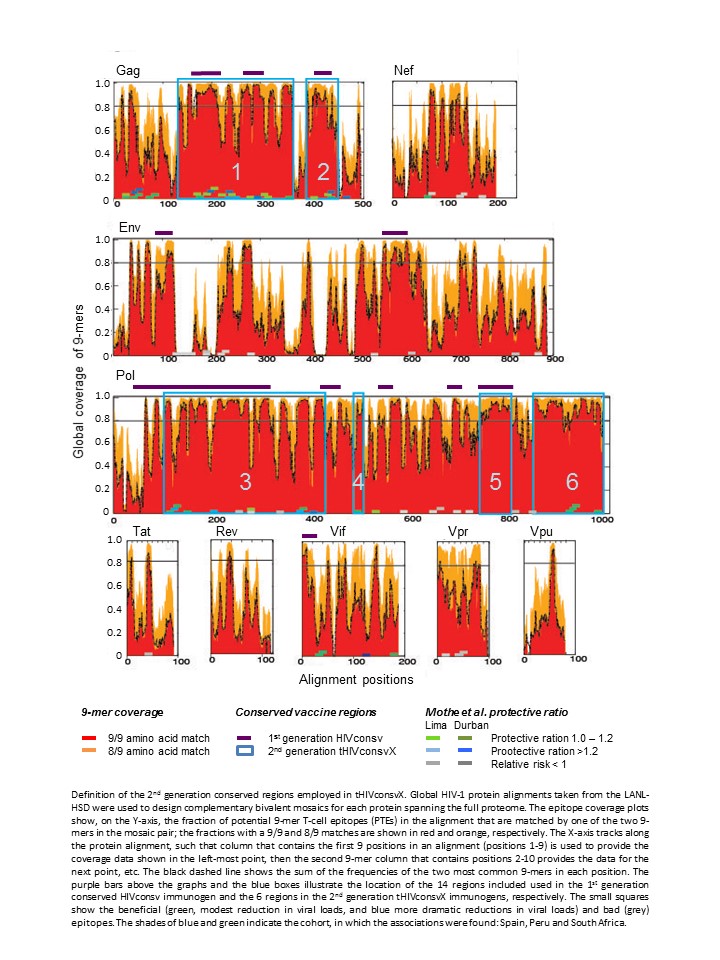
A novel T-cell vaccine against HIV-1 was designed to target the most conserved regions of the HIV-1 proteome.
HIV-1-derived bivalent mosaic immunogens tHIVconsvX match 80% of potential CD8 T-cell epitopes (over the included conserved regions) in global circulating HIV-1 isolates; perfect epitope match between the vaccine and viruses is important for efficient effector T-cell functions.
tHIVconsvX is delivered using heterologous combinations of plasmid DNA, simian (chimpanzee) adenovirus ChAdOx1 and poxvirus MVA.
In treatment-naïve HIV-1-infected patients, the presence of T cells recognizing tHIVconsvX-derived peptides correlated directly to CD4 counts and inversely to viral loads .
Abstract
An effective HIV-1 vaccine is the best solution for halting the AIDS epidemic. Here, we describe the design and preclinical immunogenicity of T-cell vaccine expressing novel immunogens tHIVconsvX, vectored by DNA, simian (chimpanzee) adenovirus and poxvirus MVA, a combination highly immunogenic in humans. The tHIVconsvX immunogens combine the three leading strategies for elicitation of effective CD8+ T cells: use of regions of HIV-1 proteins functionally conserved across all M group viruses (to make HIV-1 escape costly on viral fitness), inclusion of bivalent complementary mosaic immunogens (to maximize global epitope matching and breadth of responses, and block common escape paths), and inclusion of epitopes known to be associated with low viral load in infected untreated people (to induce field-proven protective responses). tHIVconsvX was highly immunogenic in two strains of mice. Furthermore, the magnitude and breadth of CD8+ T-cell responses to tHIVconsvX-derived peptides in treatment-naive HIV-1+ patients significantly correlated with high CD4+ T-cell count and low viral load. Overall, the tHIVconsvX design, combining the mosaic and conserved-region approaches, provides an indisputably better coverage of global HIV-1 variants than previous T-cell vaccines. These immunogens delivered in a highly immunogenic framework of adenovirus prime and MVA boost are ready for clinical development.