- HOME
- Education
- Student's Voice
- 【IRCMS Internship】Ms. Shreya Kulkarni(Indian Institute of Science Education and Research, Pune, Indi...
Student's Voice

Name: Shreya Kulkarni
Indian Institute of Science Education and Research, Pune, India
Visiting Period: December 4th (Mon), 2023 - January 19th (Fri), 2024
Country: India
Lab: Laboratory of Developmental Morphogenesis
I had the privilege of doing a 7-week internship at the International Research Center for Medical Sciences (IRCMS) in Kumamoto under the guidance of Prof. Guojun Sheng. Prof Sheng's lab works primarily on understanding Developmental processes using the chick model. Among the various projects, a prominent area of investigation revolves around comprehending the Chorioallantoic membrane (CAM) in chicken embryos.
This project, central to the lab's research, aims to deepen our understanding of developmental processes. My internship involved an in-depth exploration of the CAM, contributing to the knowledge of avian embryonic development.
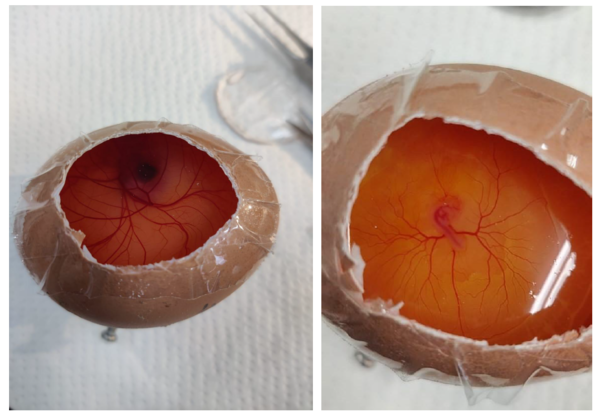
Chorioallantoic Membrane (CAM)
The Chorioallantoic membrane is a vital structure found in avian (bird) eggs, serving essential functions during the incubation period. It is formed by the fusion of the chorion and the allantois and plays a crucial role in gas exchange, nutrient transfer, and waste elimination, supporting the development and survival of avian embryos.
Studying the Chorioallantoic membrane (CAM) is valuable for several reasons:
Vascularization Studies: CAM provides a model for studying angiogenesis, especially relevant in cancer research.
Tumor Growth and Metastasis: CAM supports tumour growth, offering insights into cancer biology and drug testing.
Wound Healing and Regeneration: CAM's regenerative capacity allows the study of tissue repair and growth factor effects.
Genomic and Molecular Studies: CAM aids in investigating gene expression patterns and molecular mechanisms during development.
Embryonic Development: CAM's role in avian embryonic development provides insights into growth and nutrient exchange.
The Chorioallantoic membrane (CAM) in avian embryos is formed through the collaborative efforts of the three primary germ layers: endoderm, mesoderm, and ectoderm. During gastrulation, cells from these layers contribute to the CAM's structural and functional development. The mesoderm is particularly instrumental in creating a dense vascular network
within the CAM, supporting essential processes like gas exchange, nutrient transport, and waste elimination. This unique structure serves as a valuable model system in research, making it accessible for studies on angiogenesis, tumour growth, and various physiological phenomena.
In Situ Hybridization (Prox1 Probe):
● Prox1
Prox1 is a master regulator gene that plays a pivotal role in the development and maintenance of lymphatic vessels, a process known as lymphangiogenesis.
● During embryonic development, Prox1 is crucial for specifying lymphatic endothelial cell fate, promoting the differentiation of precursor cells into lymphatic endothelial cells.
● Prox1 is considered a key transcription factor that activates genes associated with
lymphatic vessel development, such as VEGFR-3 (vascular endothelial growth factor receptor-3) and Podoplanin.
-Probed for Prox1 gene expression within the CAM tissue.
-Enabled precise localization of Prox1 mRNA, providing insights into gene activity.
-Valuable for understanding transcriptional patterns and identifying regions of gene expression.
Results:
The CAM Tissue for Day 8, 9, 10, 12, 13, 14, 15 were probed for prox1.
There was patchy and non-specific staining, and hence, the results obtained were not reliable.
Antibody DAB Staining (Laminin, VE-Cadherin, Vimentin):
Utilized immunohistochemistry with DAB staining for Laminin, VE-Cadherin, and Vimentin.
● Allowed visualization of protein distribution within the tissue samples.
● Laminin staining highlighted extracellular matrix structures.
● VE-Cadherin staining identified vascular mesothelial cells.
● Vimentin staining revealed intermediate filament proteins.
● Provided a detailed view of protein localization, complementing the gene expression data obtained from in situ hybridization.
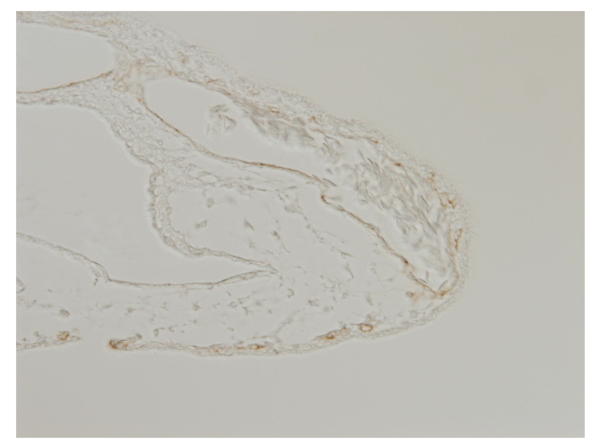
CAM Tissue at 8Day stage stained with VE-Cadherin
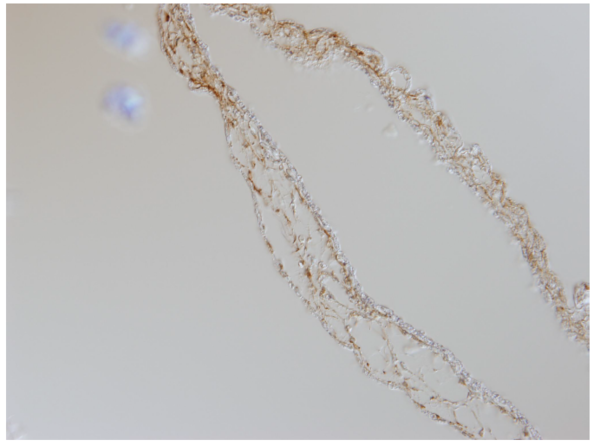
CAM tissue on Day 8 stage stained with Vimentin
I acquired a diverse skill set during my internship, including proficiency in slide mounting through meticulous techniques, preparing paraffin blocks for histological studies, and adeptly handling chicken eggs and embryos. I gained practical experience in basic cell culture techniques, in media changes and split cultures. Moreover, I engaged in other experiments involving the transplantation of human cancer cells onto chicken embryos.
These hands-on experiences have significantly broadened my technical capabilities and deepened my understanding of various laboratory procedures, enhancing my proficiency in diverse aspects of biological research.