- HOME
- Education
- Student's Voice
- 【IRCMS Internship】Ms. Rina Iwata (The University of Chicago)
Student's Voice

Name: Rina Iwata
The University of Chicago
Visiting Period: August 20th (Sun) - September 21st (Thu), 2023
Country: United States of America
Lab: Laboratory of Stem Cell Regulation
Investigation of Plcl1 Function in Hematopoiesis
Introduction
Hematopoietic stem cells (HSCs) are typically dormant--meaning they do not actively divide nor differentiate--in order to maintain their unique potential. Upon stress induction, however, HSCs begin to actively produce differentiated cells, contributing to the restoration of blood homeostasis. Whilst the precise mechanism by which dormant/active states are regulated in HSCs remains largely unknown, recent research has revealed that calcium signaling plays a crucial role. For instance, in their 2019 study, Fukushima et al., found that HSCs with high intracellular Ca2+ had significantly longer and less frequent divisions in vivo, as well as higher bone marrow reconstitution potential compared to low intracellular Ca2+ HSCs (1). Further, gene expression patterns of high and low intracellular Ca2+ HSCs appeared distinct (Tanaka, unpublished)-- including that of the Phospholipase C Like 1 (Plcl1) gene, highly expressed in HSCs with elevated intracellular Ca2+.
To better understand the regulatory mechanism of HSC state transitions, we sought to characterize the function of Plcl1--yet to be extensively investigated in hematopoiesis--using the Plcl1 KO mice model. Previously, it was found that Plcl1 is similarly enriched in platelet-biased HSCs (Tanaka, unpublished); therefore, we were particularly interested in whether Plcl1 is implicated in the maintenance of platelet homeostasis. We found that Plcl1 KO mice undergo rapid platelet (PLT) recovery around 3 days after induction of thrombocytopenia (via anti-Cd42b injection)--whilst recovery takes around 9 days in WT mice. However, the levels of actively dividing HSCs did not significantly differ between WT and Plcl1 mice 24 hours after anti-Cd42b injection. Further, WT and Plcl1 KO mice derived HSCs had comparable bone marrow homing potential. We postulate that 1) Plcl1 may play a role in the inhibition of platelet-biased differentiation more than 24 hours after platelet depletion and 2) that the Plcl1 gene may be insufficient to produce the high bone reconstitution potential seen in HSCs with elevated intracellular Ca2+.
Materials and Methods
Complete Blood Count (CBC)
- For two weeks following anti-Cd42b injection in WT (n=3) and Plcl1 KO (n=3) mice, peripheral blood was collected from the tail and analyzed for WBC, RBC, HGB, HCT, MCV, MCH, MCHC, and PLT levels.
Cell Cycle Analysis
- 24 hours after anti-Cd42b injection, HSCs from WT (n=3) and Plcl1 KO (n=3) mice bone marrow (tibia, femurs, humeri, and pelvis) were analyzed for cell cycle stage via FACS using anti-lin, anti-Cd48, anti-Cd150, anti-Cd117, anti-ScaI, and anti-Ki67 fluorescent conjugated antibodies as well as DAPI dye.
Homing Assay
- HSPCs were collected from WT (n=1) and Plcl1 KO (n=1) mice bone marrow (tibia, femurs, humeri, and pelvis) via magnetic-activated cell sorting (MACS) using anti-Cd117 magnetic beads and fluorescence-activated cell sorting (FACS) using antilineage antibody cocktail (anti-Cd3, B220, TER119, Gr1, Mac1), anti-Cd48, anti-Cd150, anti-Cd117, and anti-ScaI fluorescent conjugated antibodies. Collected WT and Plcl1 KO derived cKit+, Sca1+ Lin-(KSL) cells were then stained with CytoTell Red and injected into WT mice (n=3 mice transplanted with WT mice derived KSLs; n=3 mice transplanted with Plcl1 KO derived KSLs; 15,000 cells/mouse). 16 hours after transplantation, the amount of donor derived HSCs present in the recipient mice bone marrow (tibia, femurs, humeri, and pelvis) was analyzed with FACS.
Results
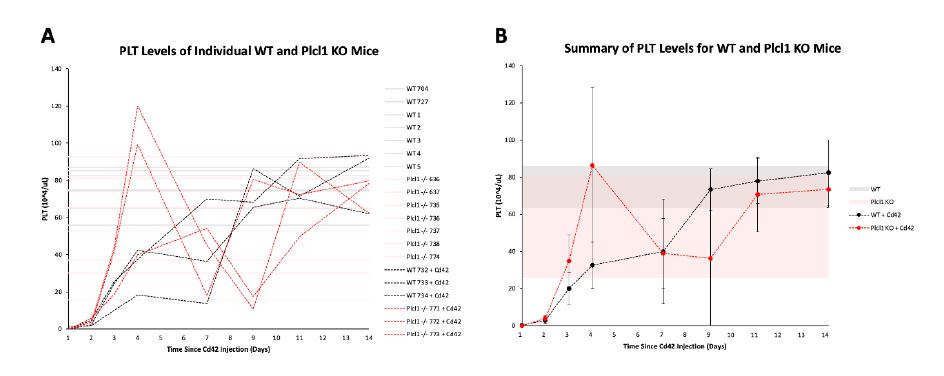
In order to investigate Plcl1 function in the maintenance of platelet homeostasis, we first injected WT and Plcl1 KO mice with anti-Cd42b--which promotes PLT aggregation and subsequent thrombocytopenia--and monitored their blood cell count for 14 days (Fig. 1 A, B). We found that in Plcl1 KO mice, PLT levels initially rapidly recover (around day 3 - 4) but drop again (around day 7 - 9) before fully recovering to levels comparable with those of WT mice injected with anti-Cd42b. However, even in Plcl1 KO mice untreated with anti-Cd42b, PLT levels had high variability (anywhere from around 20 to 80 x 10^4/uL) compared to WT mice (consistently around 60 to 90 x 10^4/uL). Because we only performed one complete blood count test on untreated mice (and did not monitor their PLT levels for the 14-day duration), we are unsure whether the fluctuations observed are caused by anti-Cd42b treatment or are an inherent consequence of depleting Plcl1.
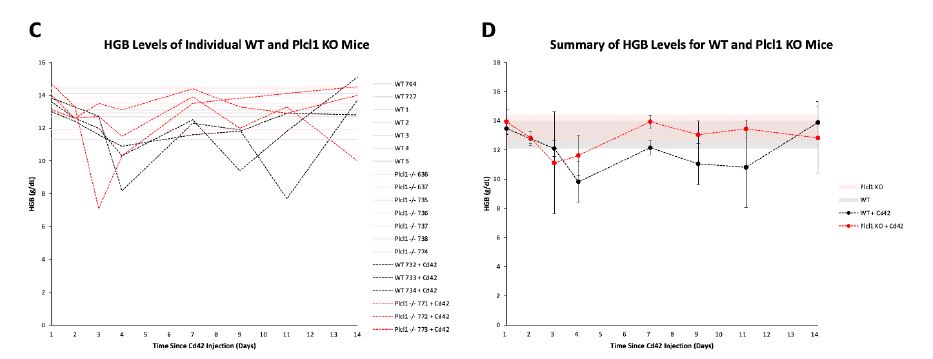
Interestingly, hemoglobin (HGB) recovery also seemed to slightly differ between WT and Plcl1 KO mice injected with anti-Cd42b (Fig. 1 C, D). Whilst both WT and Plcl1 KO mice experienced a similar decrease in HGB levels for the first 2 days after anti-Cd42b injection, WT mice appeared to undergo an additional decrease on day 4, whilst Plcl1 KO mice did not. Subsequent rate of recovery followed a similar trajectory, however, such that Plcl1 KO mice consistently had overall higher HGB levels than WT mice.
Figure 1 | PLT and HGB levels of WT and Plcl1 KO mice after anti-Cd42b injection.
Complete blood count tests were performed on 3 WT mice and 3 Plcl1 KO mice injected with anti-Cd42b (on day 0) for 14 days. Peripheral blood from 7 WT mice and 7 Plcl1 KO mice untreated with anti-Cd42 were analyzed once. A, PLT levels (10^4/uL) of individual mice on each day are shown. B, Average PLT levels of WT and Plcl1 KO mice treated with Cd42 are shown. Error bars represent standard deviation. The shaded area represents the range of PLT levels (average + SD -average - SD) measured for WT and Plcl1 KO mice untreated with Cd42. C, HGB levels (g/dL) of individual mice on each day are shown. B, Average HGB levels of WT and Plcl1 KO mice treated with anti-Cd42b are shown. Error bars represent standard deviation. The shaded area represents the range of HGB levels (average + SD - average - SD) measured for WT and Plcl1 KO mice untreated with anti-Cd42b.
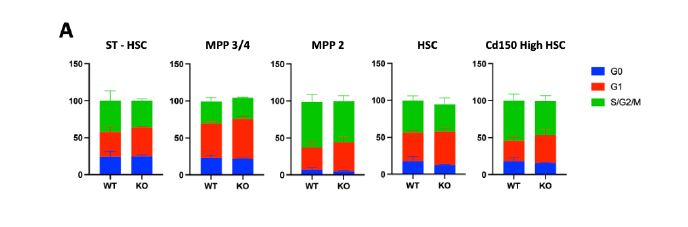
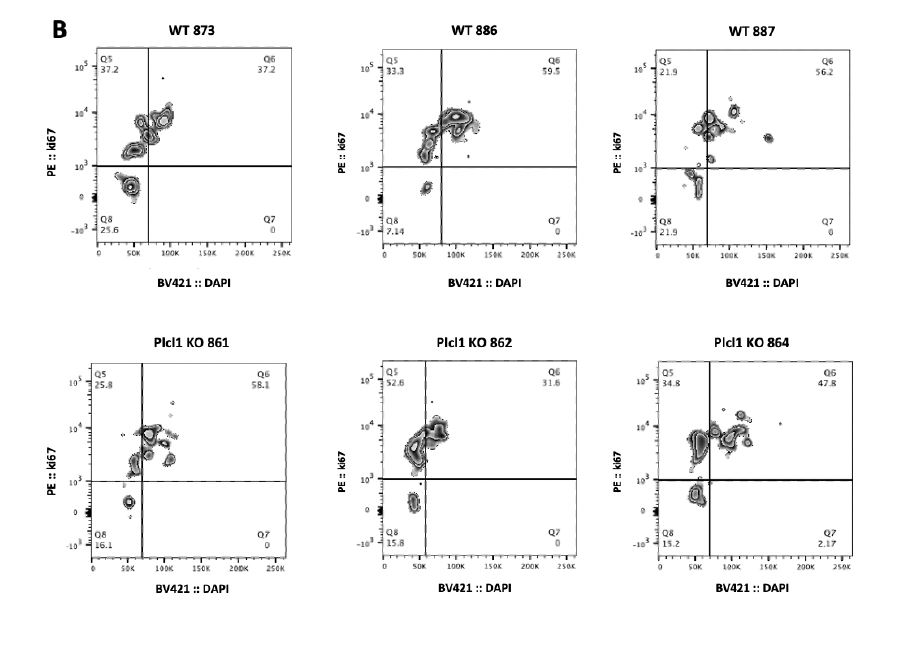
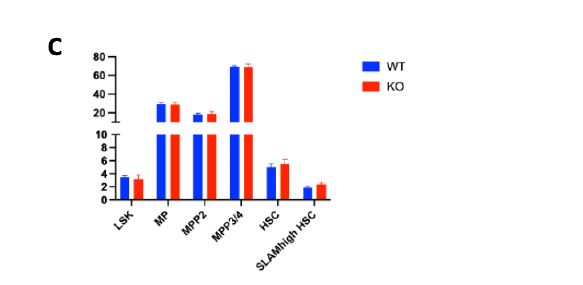
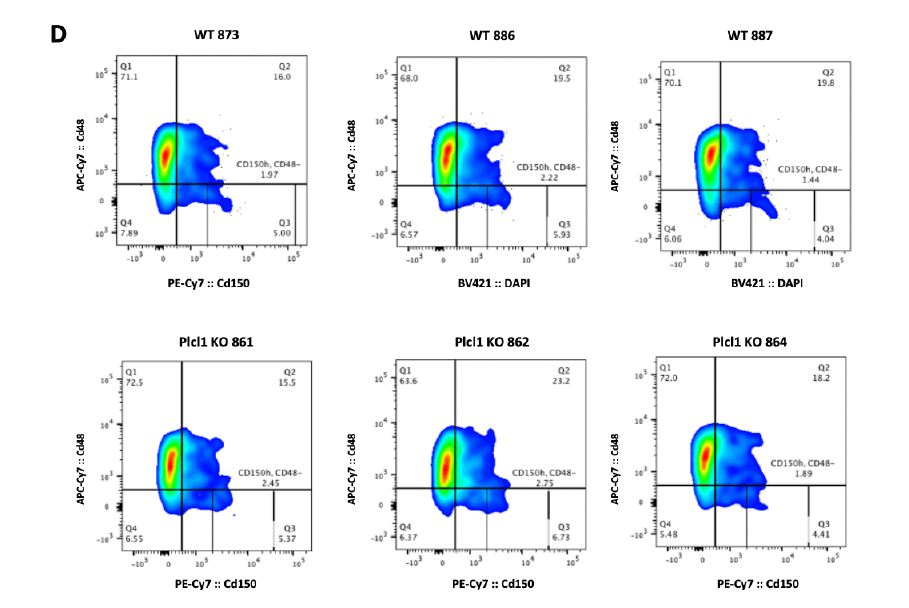
We hypothesized that the faster PLT recovery seen in Plcl1 KO mice may be due to an increase in the number of active HSCs--and therefore analyzed the cell cycle state of HSPCs from WT and Plcl1 KO mice 24 hours after anti-Cd42 injection. We found that there was a slightly greater--but not statistically significant--percentage of actively dividing HSPCs (including STHSC, MPP 3/4, MPP2, HSC, and Cd150 high HSC populations) in Plcl1 KO mice (Fig. 2 A, B). Similarly, HSC and SLAM high HSC levels were slightly elevated in Plcl1 KO mice (Fig. 2 C, D).
Figure 2 | Cell cycle state analysis of WT and Plcl1 KO mice HSPCs 24 hours after anti-
Cd42b injection. Bone marrow from 3 WT and 3 Plcl1 KO injected with anti-Cd42b were
collected and analyzed via FACS. A, Quantification of HSPC (ST-HSC, MPP 3/4, MPP 2, HSC, Cd150 HSC) cell cycle stage. B, Raw FACS data (BV :: DAPI; PE :: ki67) of the Cd150 high HSC population, which contains platelet-biased HSCs. Q5 (upper left) represents G1, Q6 (upper right) represents S/G2/M, and Q8 (bottom left) represents the G0 stage. C, Quantification of HSPC population levels. D, Raw FACS data (PE-Cy7 :: Cd150; APC-CY7 :: Cd48) of the cKit+, ScaI+, Lin- HSC population. Q2 represents the Cd150+ and Cd48+ population, which contains platelet biased HSCs.
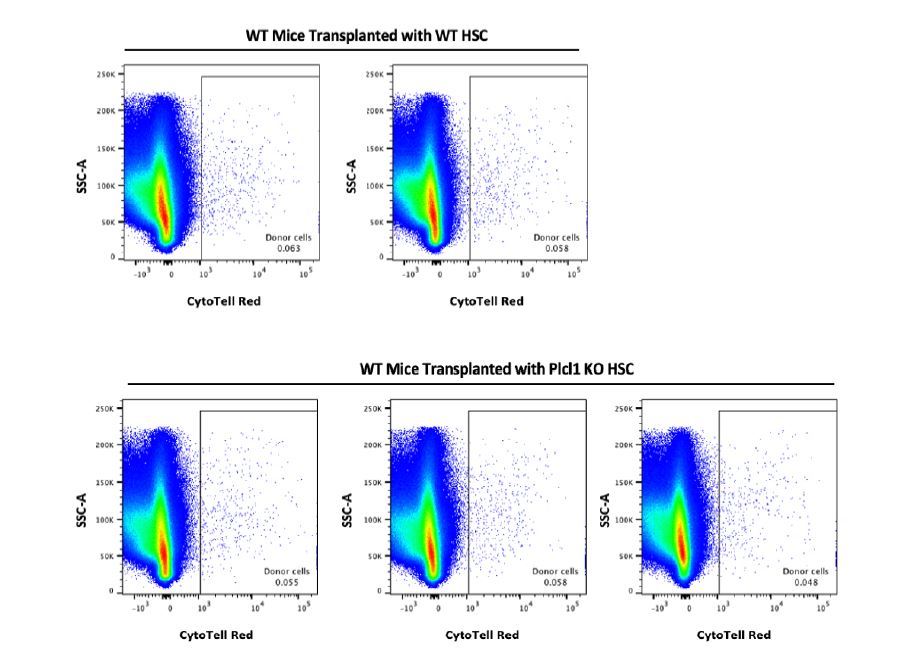
Plcl1 is not only highly expressed in platelet-biased HSCs, but also in HSCs with high intracellular Ca2+-- which have significantly longer and less frequent divisions in vivo, as well as higher bone marrow reconstitution potential compared to HSCs with low intracellular Ca2+. Plcl1 function in hematopoiesis has been not investigated and is therefore completely unknown. It has been reported that Plcl1 may have an important role in cell migration. Because Plcl1 KO HSCs showed lower bone marrow reconstitution ability than WT HSCs, we hypothesized that Plcl1 KO HSCs may not be homing to the marrow well after the transplantation. Therefore, we sought to investigate whether depletion of Plcl1 in donor HSCs affects hematopoietic chimerism in recipient mice bone marrow 16 hours after transplantation. We found, however, that both WT and Plcl1 KO HSCs had similar bone marrow reconstitution rates, indicating that Plcl1 KO HSCs have homing ability comparable to those of WT HSCs (Fig. 3).
Figure 3 | Homing Assay of WT mice transplanted with WT or Plcl1 KO HSCs. Bone marrow from 3 mice transplanted with WT HSCs and 3 mice transplanted with Plcl1 KO HSCs were analyzed (16 hours after transplantation). Shown are raw FACS data (CytoTell Red; SSC-A). The box encloses the Cytotell Red+ donor cell population.
Discussion
Here, we found that upon anti-Cd42b injection, Plcl1 KO mice experience faster initial platelet recovery (around 3 days after injection) and greater subsequent fluctuations in platelet levels compared to WT mice. Further, there was a slightly elevated, but not statistically significant, percentage of actively dividing HSPCs in Plcl1 KO mice. This suggests that Plcl1 KO may trigger increased platelet production (via increased dormant to active platelet-biased HSC state transformation) with a delay of around 72 hours (3 days) after platelet depletion. The temporary decrease in platelet levels following initial recover may also be explained by this increase in active platelet-biased HSCs, which could trigger exhaustion such that platelet homeostasis becomes unstable. Further, even in the absence of such a platelet depletion event, platelet homeostasis may be easily disrupted in Plcl1 mice due to the existence of a Plcl1-dependent regulatory system for platelet-biased HSC division.
Our data also suggests that the bone marrow homing potential of both WT and Plcl1 KO
HSCs are similar 16 hours after transplantation. Therefore, we conclude that depletion of the Plcl1 gene is insufficient to produce the high bone marrow reconstitution potential seen in HSCs with elevated intracellular Ca2+. The overexpression of Plcl1 in HSCs with intracellular Ca2+ may instead reflect how platelet-biased HSCs--whose dormant/active state are regulated by Plcl1--reside at the top of the HSC hierarchy and are part of the high intracellular Ca2+ population. In other words, high intracellular Ca2+ and overexpression of Plcl1 may be two independent characteristics of platelet-biased HSCs, and each contribute to redundant pathways that maintain bone marrow reconstitution potential.
Despite its high overexpression in HSCs, the role of Plcl1 in hematopoiesis is largely unknown. This study, therefore, seeks to begin to unravel its function--specifically in plateletbiased HSCs--and in the process, understand the intricate feedback system that regulates platelet homeostasis. I am curious about how chronic platelet depletion may affect the platelet-biased HSC pool, as well as potential binding partners of Plcl1, which may reveal to which external signals platelet-biased HSCs are most sensitive to.
Acknowledgements
I would also like to take this opportunity to express my immense gratitude to IRCMS and Suda Lab--including Dr. Suda, Dr. Tanaka, Dr. Yabushita, Ms. Kataoka, and Ms. Ideue--for this wonderful internship opportunity. These past four weeks, I learned so much (from mice dissection to ES cell culture and FACS) and was continually inspired by the wisdom, creativity, and passion of my mentors. I am forever indebted to IRCMS and Suda Lab, and am excited to continue applying what I learned here to my future research. I hope to visit Kumamoto again soon. Thank you very much for everything.
References
1. Fukushima, T., Tanaka, Y., Hamey, F. K., Chang, C. H., Oki, T., Asada, S., Hayashi, Y., Fujino, T., Yonezawa, T., Takeda, R., Kawabata, K. C., Fukuyama, T., Umemoto, T., Takubo, K., Takizawa, H., Goyama, S., Ishihama, Y., Honda, H., Göttgens, B., & Kitamura, T. (2019). Discrimination of Dormant and Active Hematopoietic Stem Cells by G0 Marker Reveals Dormancy Regulation by Cytoplasmic Calcium. Cell reports, 29(12), 4144-4158.e7. https://doi.org/10.1016/j.celrep.2019.11.061